Platomics IVD Assistant
Platomics automates regulatory processes and connects in vitro diagnostic manufacturers, laboratories and regulators to accelerate compliance and bring innovation to patients, faster.
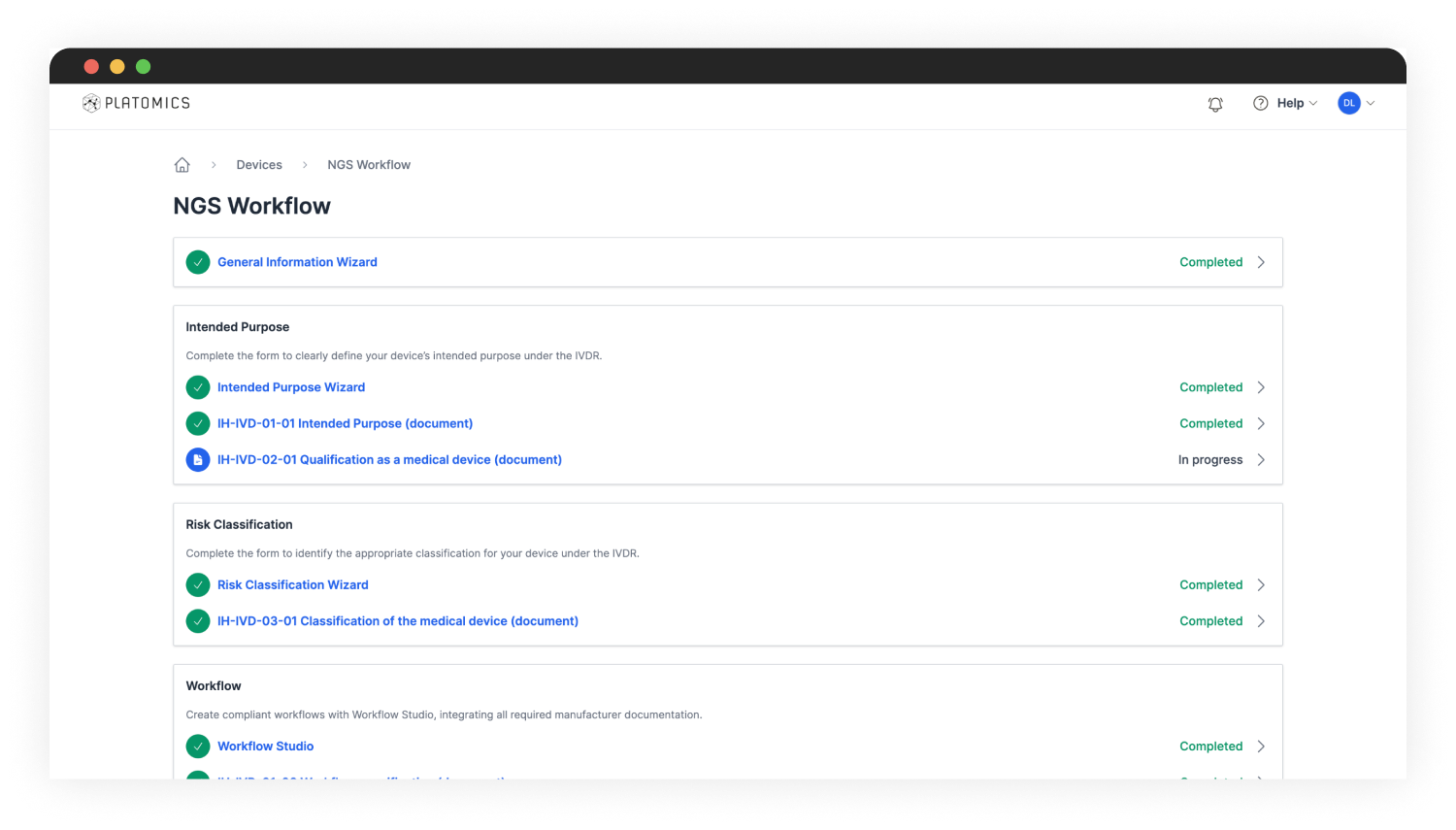
I joined Platomics’ IVD Assistant team in early 2023 and reworked the existing proof of concept to a production ready application which fulfilled the necessary step to document an in-vitro device according to the European Union’s rules coming into effect in May 2024. At the end of 2024, Platomics entered into several partnerships with device manufacturers. Among them Hamilton, Agilent Technologies, and Saphetor.
